Revealing neoantigens to generate novel anti-tumor T cell responses

2025 Nominee for Best Biomedical Product

Discovery
Candidate
IND/CTA Enabling
Phase 1
Phase 2A
Phase 2b
Investigational cancer treatment
Mechanism of action: ERAP1 inhibition
First-in-class small molecule
Therapeutic aim: reveal hidden tumors through T-cell diversification and priming
Twice daily oral solid dose
Currently in Phase 2a in combination with cemiplimab (anti-PD-1 checkpoint inhibitor)
Well tolerated with a strong safety profile

GRWD5769 alone or with cemiplimab shows good tolerability with no safety concerns
Mechanism confirmed

ERAP1 inhibition alters the immunopeptidome in a dose-dependent way, driving T-cell repertoire remodelling
Early efficacy
signals

In heavily pre-treated patients, we’re seeing confirmed partial responses and disease control lasting over 6 months, including in MSS colorectal cancer

There is a critical unmet need for cancer patients.
Despite the success of checkpoint inhibitors, around 70% of patients with many solid tumor types do not respond.
This resistance is often driven by immunologically “cold” tumors, characterized by poor T-cell infiltration, limited antigen presentation, and an immunosuppressive microenvironment.
Even in “hot” tumors with T-cell presence, chronic antigen exposure and inhibitory signaling frequently lead to T-cell exhaustion and treatment failure.
The opportunity
Our approach shows the potential to transform “invisible” tumors into immunologically engaged ones and extend benefit to the many patients who are currently left behind.
Treatments capable of broadening T-cell diversity and re-sensitizing tumors to immune attack could redefine the reach of immunotherapy.
GRWD5769 shows promise in meeting that need - aiming to awaken new T-cell responses against the toughest tumor environments.
How ERAP1 inhibition could increase tumor killing by T cells
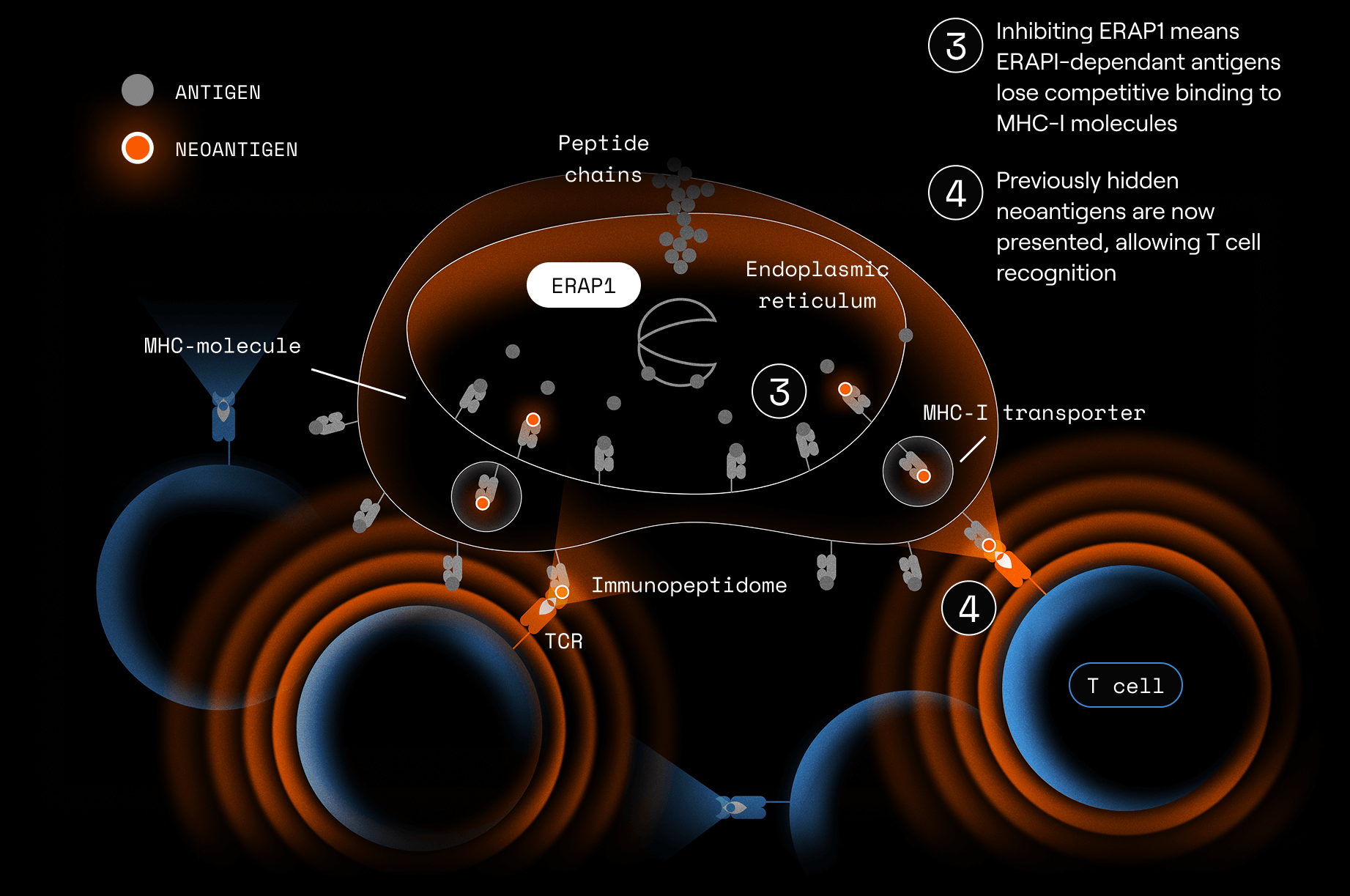
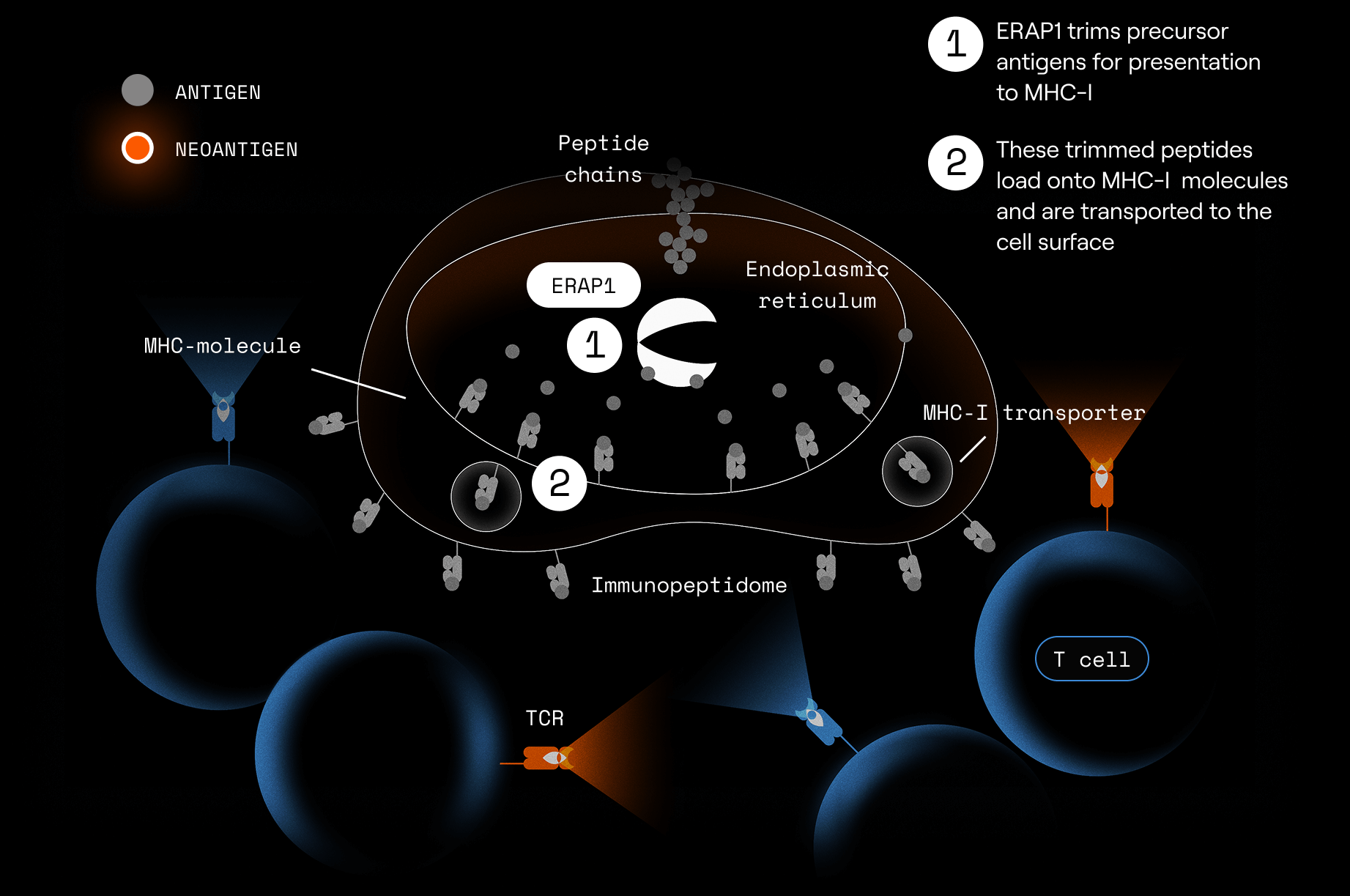
ERAP1 (Endoplasmic Reticulum Aminopeptidase 1) is a vital part of the antigen presentation pathway. It trims peptide precursors to the right length for loading onto MHC-I molecules for presentation as antigens to CD8+ T cells.
When ERAP1 is inhibited, peptides of non-standard lengths or sequences appear on MHC-I.
This can create novel or more immunogenic peptides on tumor cells, which:
- Are recognized as abnormal
- Can stimulate stronger CD8⁺ T-cell responses
- Make tumor cells more visible to the immune system
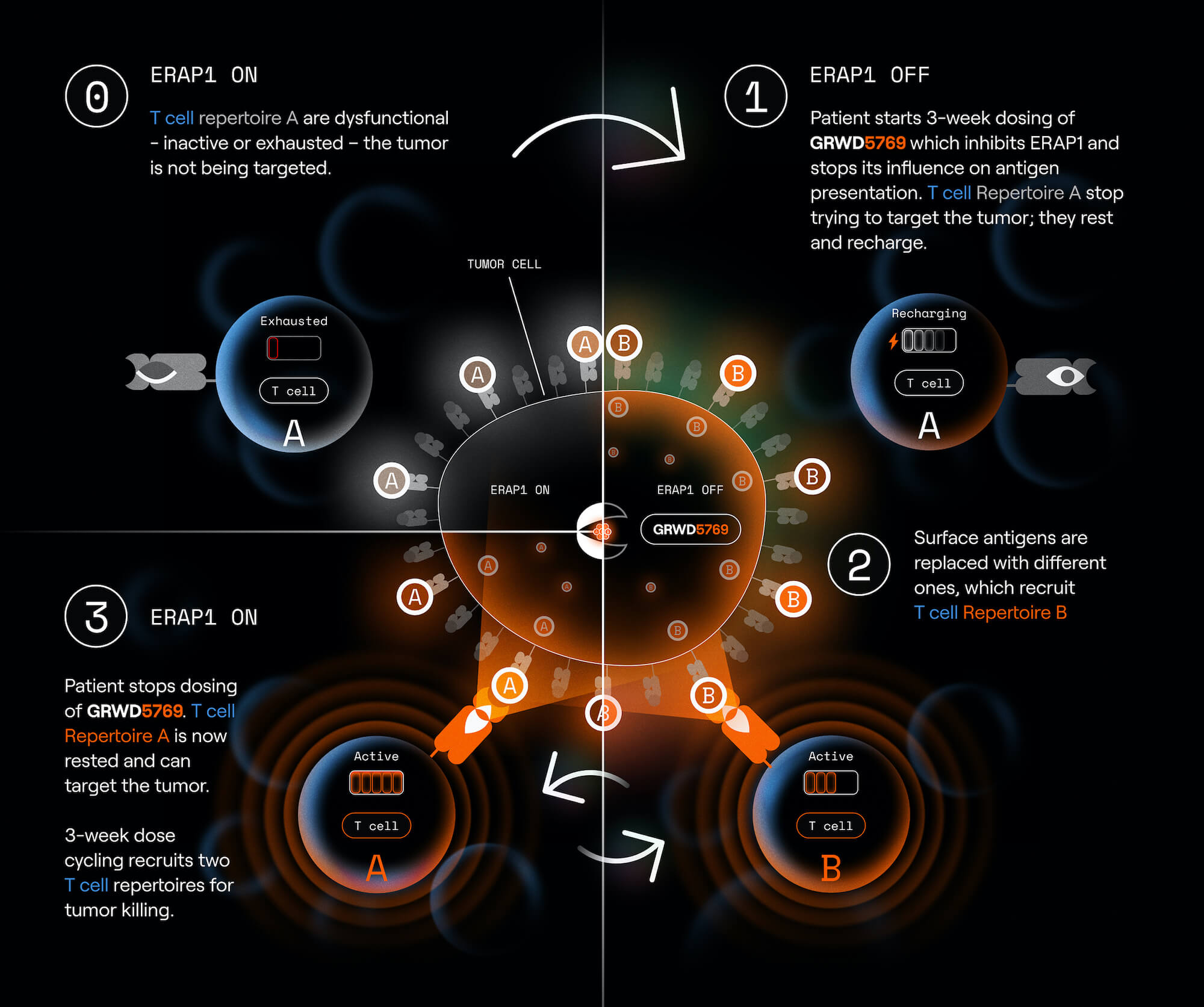
GRWD5769 both rests and recruits T cells
ERAP1 inhibition cycling replaces tumor surface antigens to cycle T cell repertoires
- Re-sensitizes tumors after acquired resistance, enabling renewed responses to checkpoint inhibitor rechallenge
- Potential to improve response to first-line immunotherapy by:
- Preventing T cell exhaustion from occurring
- Expanding T cell breadth and activity
Did you know?
Inhibiting ERAP1 may have potential in treating cancers, but research has also linked the target to autoimmune disorders. We are spearheading a first-of-its-kind trial to develop a treatment for axial spondyloarthritis, aiming to stop inflammation at the source by hiding the self-antigens incorrectly identified as foreign invaders by the immune system.
FAQs
Find answers to common questions to help you before reaching out.
What is GRWD5769?
GRWD5769 is a first-in-class, oral drug candidate being developed by Greywolf Therapeutics as a novel cancer immunotherapy for the treatment of advanced or metastatic solid tumors.
GRWD5769, both as monotherapy and in combination with cemiplimab, was well tolerated with no new safety signals. GRWD5769 monotherapy and combination have both been well tolerated at doses up to 800 mg BID, with no DLTs.
Immunopeptidomics shows that ERAP1 inhibition drives changes in the displayed immunopeptidome in a dose dependent manner, and that leads to diversification and remodeling of the T-cell repertoire, an important step in reawakening immune responses to tumors.
In heavily pre-treated patients, we’ve already seen confirmed partial responses (including in MSS colorectal cancer, a notoriously immune-resistant disease) and durable disease control beyond 6 months in multiple patients.
What is Signal 1?
GRWD5769 is the first treatment to manipulate Signal 1 in T cell activation.
T-cell activation requires a chain of three signals. All current approaches to treating cancer are focused on changing the later steps in the signaling cascade - Signals 2 & 3.
Greywolf Therapeutics is the first company to successfully manipulate Signal 1 (antigen recognition) and impact the initial detection of a cell by a T cell.
What is EMITT-1?
This is a Phase 1/2, open-label, first-in human study of GRWD5769 alone, and in combination with another anti-cancer agent in advanced solid cancers. More information is available on ClinicalTrials.gov (NCT06923761)
How does GRWD5769 work?
GRWD5769 is a potential new cancer treatment that works by inhibiting the enzyme endoplasmic reticulum aminopeptidase 1 (ERAP1) to make tumor cells more "visible" to the immune system. This mechanism is designed to generate a new anti-tumor immune response and help patients overcome resistance to existing immunotherapies.
What stage of development is GRWD5769 in?
Phase 1/2 trials
What makes GRWD5769 different from existing therapies?
GRWD5769 is a novel first-in-class investigational treatment, differentiated by its focus on precision modulation the Signal 1 pathway involved in antigen presentation. Rather than relying on generalized immune stimulation, it is designed to selectively influence immune recognition at the molecular level, with the potential to expand the scope of targets that the immune system can effectively address.
By changing the whole peptidome isn’t there a danger you create new self-peptides and trigger autoimmune diseases?
All “self” peptides are already present—ERAP1 inhibition alters relative abundance, i.e. ERAP1i either up-regulates or down-regulates the existing self peptides. As a result, those peptides are thymically-tolerized.
When you talk about T-cell exhaustion and switching between two antigenic states, is it the same T cells recovering, or completely new clones engaging?
Both. Cycling 3 weeks on/3 weeks off creates two non-overlapping peptide repertoires. Some previously exhausted clones can rest and re-engage; novel ERAP1i cancer antigens can recruit and activate new T clones as well.